Abstract
Introduction: Patients (pts) with TDT who require life-long regular RBC transfusions (RBCTs) are at risk for increased mortality due to iron overload. Iron unloading can take years, even with ICT use. In the phase 3 BELIEVE trial, pts with TDT receiving luspatercept experienced meaningful reductions in RBCTs.
Here, we report long-term iron parameter results for pts with TDT in the BELIEVE trial receiving luspatercept for up to 288 weeks (wk).
Methods: Pts were ≥ 18 years of age with TDT (hemoglobin [Hb] E/β-thalassemia, compound β-thalassemia mutation and/or multiplication of α-globin genes was allowed) and required regular RBCTs (6-20 RBC units in the 24 wk prior to randomization, no transfusion-free period > 35 days). Pts were randomized 2:1 to receive luspatercept (1.0-1.25 mg/kg) or placebo (PBO) subcutaneously every 3 wk. All pts could receive RBCTs to maintain Hb levels, as well as ICT. Data for PBO pts are presented up to wk 96 as the majority had crossed over to receive luspatercept by this time.
Mean change from baseline (BL) in RBC units transfused (BL defined as RBCT burden in the 24 wk prior to study × 2), ICT dose and serum ferritin (SF) level (BL calculated during the 12 wk prior to first dose), liver iron concentration (LIC) and myocardial iron (BL calculated during last assessment on/before first dose) were calculated using only BL values from pts assessed at each time point. Proportions of pts who shifted from BL SF level ≥ 1000 μg/L to < 1000 μg/L and from BL LIC ≤ 3 mg/g dw to > 15 mg/g dw were also assessed. Luspatercept responders were defined as pts who achieved ≥ 33% or ≥ 50% reduction in RBCTs up to data cutoff vs BL during any 12 or 24 wk.
Results: Overall, 224 pts were randomized to luspatercept and 112 to PBO; 92 PBO pts crossed over to receive luspatercept ("crossover"). As of Jan 15, 2022, median (range) treatment duration was 229.1 (1.7-285.9) wk for luspatercept, 176.5 (6.1-180.4) wk for crossover, and 74.7 (8.9-104.0) wk for PBO. At wk 144, 58.9% of luspatercept and 63.0% of crossover pts completed treatment; approximately 50% of pts randomized to receive luspatercept completed 240 wk of treatment.
Mean change from BL in RBC units transfused at the latest available time points was −6.16 for luspatercept (wk 193-240; n = 102), −5.18 for crossover (wk 97-144; n = 56), and +0.31 for PBO (wk 49-96). Mean reductions in RBCTs ranged from 4.72-6.37 units for luspatercept and 5.05-5.75 units for crossover pts over the entire treatment period.
In luspatercept pts, mean daily ICT dose declined from BL through wk 192 (deferasirox: −231.7 mg [n = 51]; deferiprone: −278.0 mg [n = 15]; deferoxamine: −214.3 mg [n = 3]); reductions were more pronounced in responders. Crossover pts experienced similar, though less pronounced, reductions in deferasirox mean daily dose at wk 96 (−242.1 mg [n = 29]).
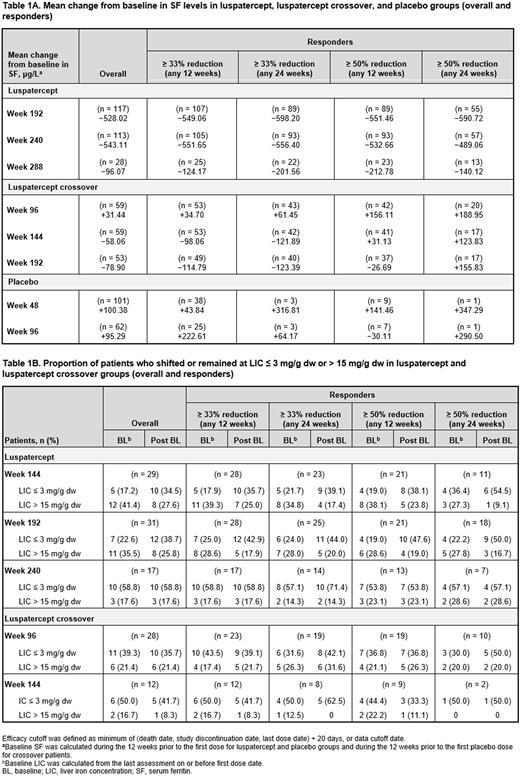
Mean change from BL in SF level (μg/L) was −528.02 at wk 192 (n = 117), −543.11 at wk 240 (n = 113), and −96.07 at wk 288 (n = 28) in luspatercept; −58.06 at wk 144 (n = 59) and −78.90 at wk 192 (n = 53) in crossover; and +95.29 at wk 96 (n = 62) in PBO pts. Reductions in the luspatercept arm were more pronounced in responders (Table 1A).
A considerable proportion of luspatercept pts with BL SF level ≥ 1000 μg/L shifted to < 1000 μg/L at wk 192 (39.5%), wk 240 (45.9%), and wk 288 (44.4%). At wk 144 and 192, 33.3% and 38.7% of crossover pts, respectively, shifted to lower SF level; 8.9% and 11.1% of PBO pts at wk 48 and 96, respectively, experienced similar shifts.
Mean change from BL in LIC (mg/g dw) was −4.16 at wk 144 (n = 29), −1.26 at wk 192 (n = 31) and +0.13 at wk 240 (n = 17) in luspatercept; −0.03 at wk 96 (n = 28), +0.32 at wk 144 (n = 12) in crossover; and −1.32 at wk 96 (n = 5) in PBO pts. Proportions of luspatercept pts with LIC ≤ 3 mg/g dw tended to increase and those with LIC > 15 mg/g dw tended to decrease compared to BL (Table 1B). No meaningful shifts in LIC categories in crossover pts were observed, although the analysis was limited by small pt numbers.
Most pts had normal myocardial iron T2* levels (> 20 ms) at BL and all evaluable time points.
Conclusions: Results from this analysis are consistent with previous time points. Pts with TDT receiving long-term luspatercept treatment continued to experience sustained and durable reductions in RBCT burden, ICT use, and SF level over time. Luspatercept also resulted in decreases or stabilization of LIC starting at wk 96. Improvement or stabilization of iron-related parameters by luspatercept may support better longer-term outcomes for pts with TDT.
Disclosures
Hermine:Kite/Gilead: Honoraria; Novartis: Research Funding; BMS: Honoraria, Research Funding; Inatherys: Research Funding; AB Science: Current equity holder in private company, Honoraria, Research Funding. Cappellini:Sanofi Genzyme: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Silence: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Vertex: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Taher:Ionis Pharmaceuticals: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Imara: Consultancy, Research Funding; Vifor Pharma: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Coates:Forma: Consultancy; Chiesi: Consultancy; Bluebird: Consultancy; Vifor: Consultancy; BMS Pharma: Consultancy; Agios: Consultancy. Kattamis:VERTEX: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria, Research Funding; Chiesi: Honoraria; Novartis: Consultancy, Honoraria, Research Funding; ViFOR: Consultancy; AMGEN: Consultancy; AGIOS Pharmaceuticals: Consultancy; IONIS: Consultancy. Shetty:Celgene/Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Bosilkovska Weisskopf:BMS: Current Employment, Current holder of stock options in a privately-held company; Vifor Pharma: Other: Husbands current employer. Holot:BMS: Current Employment. Vodala:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Kuo:Bristol-Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Porter:BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Protagonism: Honoraria; La Jolla Pharmaceuticals: Honoraria; Celgene: Consultancy, Honoraria; bluebird bio: Consultancy, Honoraria; VIFOR: Honoraria; Agios: Consultancy, Honoraria; Silence Therapeutics: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal